
Ryder System Inc. (R)
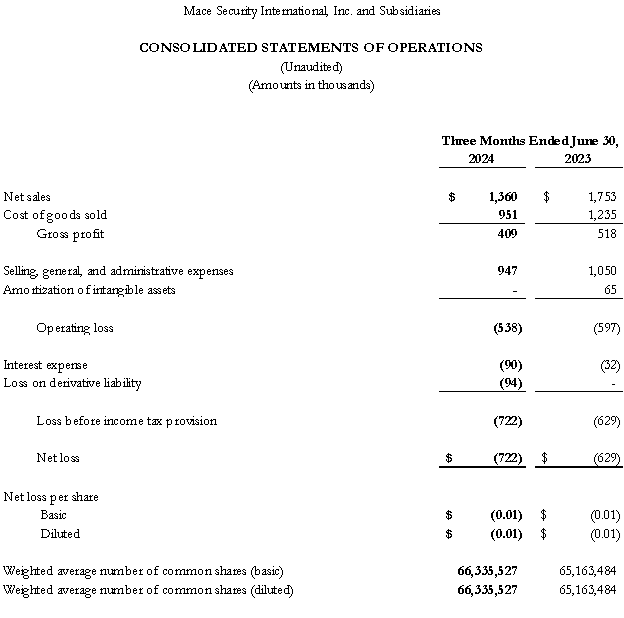
Mace(R) Security International, a Global Leader in Personal Self-Defense Sprays, Announces 2Q24 Financial Results
CLEVELAND, OH / ACCESSWIRE / September 30, 2024 / Mace Security International (OTCQB:MACE) today announced its second quarter 2024 financial results for the period ended June 30, 2024. Second Quarter 2024 Financial Highlights The Company's net sales for the second quarter of 2024 were $1,360,000, down (22%) versus the like period in 2023.

Mace(R) Security International, a Global Leader in Personal Self-Defense Sprays, Announces Additional Unsecured Subordinated Funding
CLEVELAND, OH / ACCESSWIRE / September 26, 2024 / Mace Security International (OTCQB:MACE) today announced that the Company's Board of Directors approved on September 20, 2024 a $40,000 unsecured subordinated loan from a Board member. The note matures on the sooner of July 27, 2025 or when the Company's senior line of credit is repaid.

Theralase(R) Closes Non-Brokered Private Placement and Issues Stock Options
TORONTO, ON / ACCESSWIRE / September 24, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it has successfully closed a Non-Brokered Private Placement ("NBPP") offering ("Offering") of units ("Units"). On closing, the Corporation issued an aggregate of 2,720,000 Units at a price of $CAN 0.20 per Unit for aggregate gross proceeds of $CAN 544,000.

Just - Evotec Biologics Opens Cutting-Edge Biologics Facility - J.POD(R) Toulouse, France (EU)
J.POD® Toulouse, France (EU) brings disruptive, scalable continuous biologics manufacturing technology to the region Just - Evotec Biologics' J.POD® technology offers a paradigm shift in biomanufacturing with cost-effective and flexible clinical and commercial supply solutions HAMBURG, GERMANY and TOULOUSE, FRANCE / ACCESSWIRE / September 20, 2024 / Just - Evotec Biologics, the biologics segment of Evotec SE (Frankfurt Stock Exchange:EVT)(MDAX/TecDAX, ISIN: DE0005664809)(NASDAQ:EVO), today celebrated the Grand Opening of its J.POD® biologics development and manufacturing facility located on Evotec's Campus Curie in Toulouse, France. The J.POD® facility in Toulouse, the second of its kind and the first in Europe, utilizes Just - Evotec Biologics' adaptable J.POD® technology to provide essential clinical and commercial biologics manufacturing capacity.

Nutriband Licenses Bitrex(R) Brand Aversive Agent for Its Lead Product - Aversa(TM) Fentanyl Transdermal Patch
Nutriband signs trademark licensing agreement for Bitrex® brand denatonium benzoate, the most bitter substance in the world, as an aversive agent for its Aversa™ Fentanyl abuse deterrent fentanyl transdermal patch Nutriband abuse-deterrent transdermal technology consists of a proprietary aversive agent coating that employs taste aversion to deter the oral abuse of and accidental exposure to transdermal opioid and stimulant patch products ORLANDO, FL / ACCESSWIRE / September 20, 2024 / Nutriband Inc. (NASDAQ:NTRB)(NASDAQ:NTRBW), a company engaged in the development of prescription transdermal pharmaceutical products, today announced that it has signed a trademark licensing agreement for the use of Bitrex® brand denatonium benzoate as an aversive agent in its lead product, AVERSA™ Fentanyl, an abuse deterrent fentanyl patch. Nutriband's AVERSA™ abuse-deterrent technology can be utilized to incorporate aversive agents into transdermal patches to prevent the abuse, diversion, misuse, and accidental exposure of drugs with abuse potential.

Theralase(R) Extends Warrants
TORONTO, ON / ACCESSWIRE / September 19, 2024 / Theralase® Technologies Inc. ("Theralase" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that the Company proposes to extend the expiry date of 10,000,000 share purchase warrants ("Warrants") from September 22, 2024 to September 22, 2027. The Warrants were issued on September 22, 2022, pursuant to a private placement involving the issuance of 10,000,000 units of the Company.

Levi & Korsinsky, LLP Announces $45,000,000 Recovery for Ryder System, Inc. (R). File Your Claim Before Deadline
NEW YORK, NY / ACCESSWIRE / September 5, 2024 / Levi & Korsinsky informs shareholders that a settlement has been reached in the pending class action lawsuit against Ryder System, Inc. (NYSE: R). The settlement provides for a fund of $45,000,000 to benefit class members.

Time Sensitive - Reminder from Levi & Korsinsky, LLP to File Claim for Share of Ryder System, Inc. (R) Settlement Fund Before Deadline
NEW YORK, NY / ACCESSWIRE / September 10, 2024 / Levi & Korsinsky informs shareholders that a settlement has been reached in the pending class action lawsuit against Ryder System, Inc. (NYSE: R). The settlement provides for a fund of $45,000,000 to benefit class members.

Sonoma Pharmaceuticals Receives New FDA 510(k) Clearance with Expanded Indications for Over-the-Counter Microcyn(R)-Based Solution
BOULDER, CO / ACCESSWIRE / September 17, 2024 / Sonoma Pharmaceuticals, Inc. (NASDAQ:SNOA), a global healthcare leader developing and producing patented Microcyn® technology based stabilized hypochlorous acid (HOCl) products for a wide range of applications, including wound care, eye, oral and nasal care, dermatological conditions, podiatry, and animal health care, today announced it has received a new 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Microcyn technology-based solution, including specific over-the-counter indications for the face, eyelid and eyelashes. Per this new clearance, Sonoma's Microcyn wound care solution can be used for OTC management of minor skin abrasions, lacerations and irritations, and intact skin on the face, eyelid and eyelashes.

Ryder Stock Rallies 20% in 6 Months: What Should Investors Do Now?
With R shares appreciating this year, we assess the current positioning of the stock to determine if it's a good investment choice at this juncture.

BioInvent Announces the Enrollment of the First Patient in Triple Combination Arm of BI-1206, Rituximab and Calquence(R) for the Treatment of non-Hodgkin's Lymphoma
First patient enrolled in Phase 2a study arm combining BI-1206 with rituximab and acalabrutinibwith initial data expected YE 2024 Clinical supply agreement for acalabrutinib with AstraZeneca in place LUND, SE / ACCESSWIRE / September 12, 2024 /BioInvent International AB ("BioInvent") (Nasdaq Stockholm:BINV), a biotech company focused on the discovery and development of novel and first-in-class immune-modulatory antibodies for cancer immunotherapy, today announces it has enrolled the first patient in the triple combination arm of the Phase 1/2a study of its anti-FcgRIIB antibody, BI-1206 in non-Hodgkin's lymphoma (NHL). The Phase 2a study arm will combine the subcutaneous formulation of BI-1206 and rituximab with Calquence® (acalabrutinib), a selective inhibitor of Bruton's tyrosine kinase (BTK).

Petros Pharmaceuticals Executes Successful Initial Test for App Comprehension as Part of FDA Pathway for Over-the-Counter Access for STENDRA(R) (avanafil)
Company is currently conducting larger scale comprehension test to confirm results in compliance with FDA discussions NEW YORK, NY / ACCESSWIRE / September 11, 2024 / Petros Pharmaceuticals, Inc. (NASDAQ:PTPI) ("Petros" or the "Company"), a company focused on expanding consumer access to medication through over-the-counter ("OTC") drug development programs, today announced results of an initial study to determine consumer comprehension of the messaging in its App Technology ("App Comp"), which has unique differences from the current Drug Facts Label ("DFL"). Of the 31 objectives, 29 scored >90% comprehension Point Estimate ("PE"), 30 scored >86.7% comprehension PE, and all scored > 80% comprehension PE.







