
Merck & Co., Inc. (MRK)

Gardasil Worries Loom as Merck Reports Earnings
Merck shares are down more than 20% over the past 12 months after trouble with sales of its Gardasil vaccine in China
MRK Gets Positive CHMP Nod for 21-Valent Pneumococcal Jab Capvaxive
The Committee for Medicinal Products for Human Use recommends approval for Merck's pneumococcal 21-valent conjugate vaccine, Capvaxive.

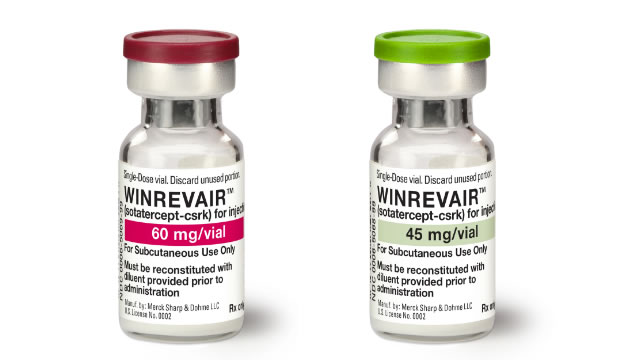
Merck Halts Second PAH Drug Study Early on Strong Efficacy Results
MRK's decision to stop the late-stage HYPERION study on PAH drug Winrevair ahead of time comes after evaluating the drug's overall clinical program data.
Unveiling Merck (MRK) Q4 Outlook: Wall Street Estimates for Key Metrics
Looking beyond Wall Street's top -and-bottom-line estimate forecasts for Merck (MRK), delve into some of its key metrics to gain a deeper insight into the company's potential performance for the quarter ended December 2024.
Merck & Co., Inc. (MRK) is Attracting Investor Attention: Here is What You Should Know
Merck (MRK) has received quite a bit of attention from Zacks.com users lately. Therefore, it is wise to be aware of the facts that can impact the stock's prospects.
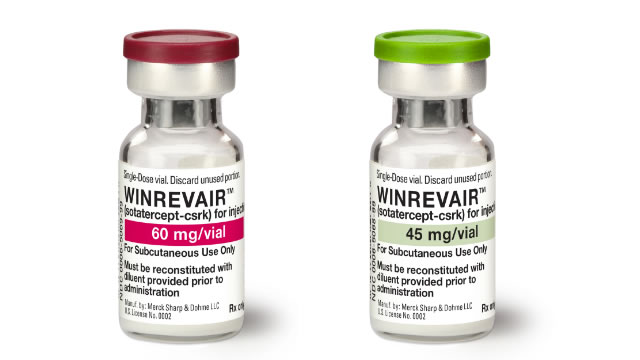
Merck to stop lung condition drug study early due to strong efficacy data
Merck plans to stop a late-stage study testing its drug to treat pulmonary arterial hypertension (PAH) ahead of time based on strong efficacy shown in previous studies, the drugmaker said on Thursday.

MRK's sNDA for Welireg in Rare Tumors Gets FDA's Priority Tag
The FDA accepts and grants priority review to Merck's sNDA for Welireg to treat advanced pheochromocytoma and paraganglioma. A decision is due on May 26, 2025.
Merck (MRK) Earnings Expected to Grow: Should You Buy?
Merck (MRK) doesn't possess the right combination of the two key ingredients for a likely earnings beat in its upcoming report. Get prepared with the key expectations.
Merck Q4 Earnings Coming Up: Buy, Hold or Sell MRK Stock Now?
When Merck reports fourth-quarter earnings, investors are likely to focus on the sales of its blockbuster oncology medicine, Keytruda.
MRK Reports Mixed Keytruda Combo Study Data in Gastroesophageal Cancer
The LEAP-015 study is evaluating Merck's Keytruda and Eisai's Lenvima plus chemotherapy in certain types of gastroesophageal adenocarcinoma.

Why This Beaten-Down Stock Is a Buy in 2025 and Beyond
Last year wasn't a good one for Merck (MRK -1.12%), one of the largest pharmaceutical companies in the world. The healthcare giant is dealing with the fast-approaching patent cliff for its most important product, cancer medicine Keytruda.

Kennedy would keep legal fees from Merck cases if confirmed
Robert F. Kennedy Jr. would retain legal fees earned from litigation against drugmaker Merck if he is confirmed as President Donald Trump's secretary of the U.S. Department of Health and Human Services, according to a federal ethics disclosure made public on Wednesday.







